| Curcumin Many spices as well as fruits and vegetables have polyphenolic antioxidants that also have antiinflammatory acitivity. These compounds can give these food their color. We have found that the yellow pigment in turmeric, curcumin, can act at multiple steps in Alzheimer pathogeneis to stop and even reverse damage. |
Omega-3 fatty acids An dietary essential fat critical for optimal nerve cell function is frequently deficient in the American Diet and in human breast milk. This fat depletes more rapidly in Alzheimer disease brain because of oxidation, and its replenishment in animals restores many functions. The omega-3 fatty acids in plants are not easily convertable to the one that we need in our brain, docosahexaenoic acid, so dietary intake of fish and some algaes is necessary. |
| R-Flurbiprofen (FLURIZAN) Our laboratory and others have shown beneficial effects of flurizan in in vitro and in vivo models, which led to its testing in clinical trialas. Preliminary data suggests that this anti-inflammatory drug may stop progression of Alzheimer's. |
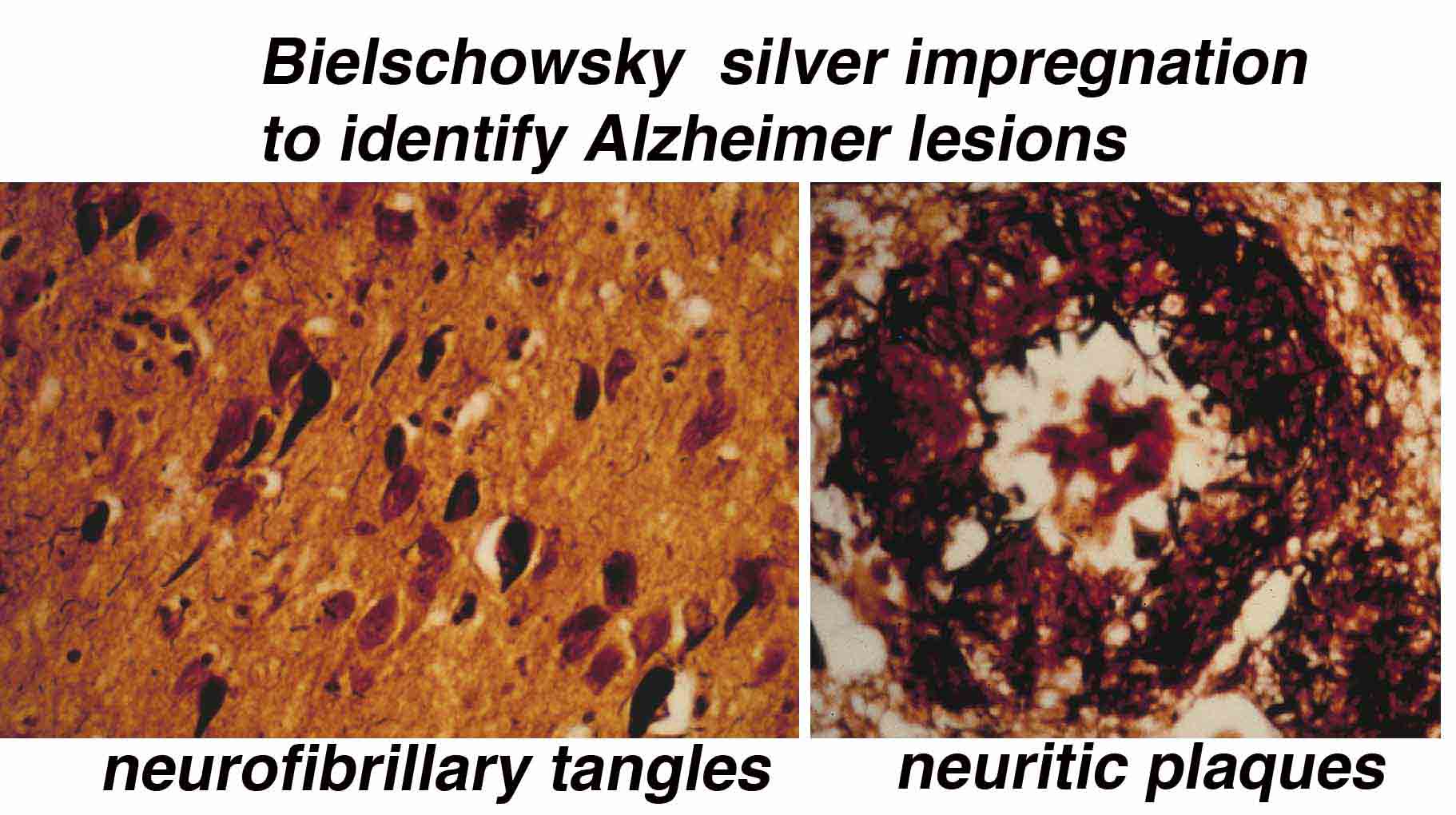
Lipoproteins & Cognition. We have found that an inflammatory substance called TGFß that is elevated in the neurofibrillary tangle lesions of Alzheimer's brains can enhance vulnerablity to the toxic amyloid, causing neuron loss. It does so by favoring its uptake by the lipoprotein related receptor on nerve cells. Cells with this receptor are lost early in the disease and may aggravates amyloid accumulation in addition to contributing to memory loss. |
| Ibuprofen Our laboratory tested the impact of ibuprofen in Alzheimer's models because of extensive epidemiology suggesting that some non-steroid anti-inflammatory drugs may reduce risk for Alzheimer's. It turned out that ibuprofen did reduce some inflammatory indices of toxicity (interleukin-1ß) and plaque burden, but not all indices (that is not oxidative damage). |
Soluble and insoluble Amyloid. There are different pools of amyloid. The undeposited or soluble aggregate form (oligomers) better predicts neurodegeneration. Our lab was one of the first labs to test oligomer toxicity in vivo, showing specific cell loss, typical of that observed prior to onset of disease (in AD vulnerable regions such as entorhinal cortex layer 2 and CA1 of hippocampus). We recently found that the particular aggregate that causes this toxicity is probably at least a 12-mer and has much more acute effects of neuron death than the lower molecular weight oligomers (dimer, trimers). |
Alzheimer's Research Mission
Statement: Our laboratory conducts research to better understand the cellular, biochemical and genetic pathways that disrupt cognition in Alzheimer's disease. Models are developed and used to investigate candidate molecules and cocktails that can prevent and even stop the disease . Emphasis is on safe treatments that have strong rationale and extensive testing at the basic research level, on compounds, so that therapies can move rapidly from bench to bedside. Our goal is to coordinate our research with the NIH National Consortium Clinical Trials and local Alzheimer centers (UCLA Alzheimer Center) to accelerate effective prevention and treatment methods for Alzheimer's disease. Members of the Team. Our group was formed in Dec 1993 by Greg Cole, PhD and Dr. Sally Frautschy when they joined UCLA and the VA GRECC. It now consists of 4 key faculty members as wells as senior post-doctoral researchers, visiting scholars and experienced technicians. Greg Cole, PhD is the team leader who has worked on Alzheimer's since 1980 at UC Berkeley. He is the Associate Director of Research at the Veterans Administration Geriatric Research Education Clinical Center and Associate Director of Research at the UCLA Alzheimer Research Center, and Professor of Medicine (& Neurology) at UCLA. Dr. Frautschy is also a Professor of Medicine (& Neurology) who has a MS & PhD in physiology and expertise designing models to mimic human diseases. Both Cole and Frautschy joined forces in 1993 to start a laboratory geared to improving treatments to reduce the incidence and prevalence of AD. Two other faculty members in this group are Drs. Bruce Teter (with expertise in apolipoproteins) and Qiulan Ma (with expertise in molecular and cell biology and beta-amyloid oligomers) and Ed Teng (MD, PhD, GRECC Neurology) . Other key members of the team are post doctoral associates (Drs. Fusheng Yang, Shuxin Hu, Aynun Begum, Atul Desphande, MD from Jorge Busciglio's lab)